EmCell Stem Cell Center provides programs for children from the age of thee years as well as for adults of any age who are able to travel to us. The main contraindications are active malignancy and active infection. We do not accept every patient, because the potential efficacy of treatment is just as important to us as safety. If we do not see a genuine possibility to help, we do not admit the patient and we never create false expectations about what stem cells can achieve. Above all, we remain committed to caring deeply for the well-being of every patient.
Decades of Extensive Experience
Successful Patient Outcomes
High-Potency Mesenchymal Stem Cells
Personalized Treatment Plan
Treatment programs
Stem cell therapy is at the forefront of medical innovation, offering new possibilities for treating a wide range of conditions. Research shows that mesenchymal stem cells can regenerate damaged tissues, reduce inflammation, and modulate the immune system—supporting better health and quality of life. We provide a personalized strategy tailored to your needs, including customized stem cell and exosome dosages, targeted delivery methods, and supportive care. Conditions commonly addressed include:
Safety & Regulations
Safety and effectiveness are our top priorities. We always follow the required regulations for the production of stem cell products and their clinical application, adhering to both local and international standards to ensure the highest quality care for our patients.
Reduce Inflammations
Ethically Sourced Tissue
95% +
Viability
GMP Cell Product
Good Manufacturing Practice (GMP) provides a structured framework to ensure the quality and safety of cell therapy products used in clinical settings. Following GMP standards guarantees that each product meets strict criteria for identity, purity, potency, and overall quality.
GMP production involves a carefully controlled and fully documented process, ensuring consistency, safety, and reliability in every batch of cells. This rigorous approach is essential not only for regulatory compliance but also for patient safety, as these “living medicines” are delivered for therapeutic use.
Our stem cell products undergo thorough safety and quality checks before, during, and after processing. These measures comply with GMP standards and involve seven rigorous stages to ensure the highest level of quality and safety.
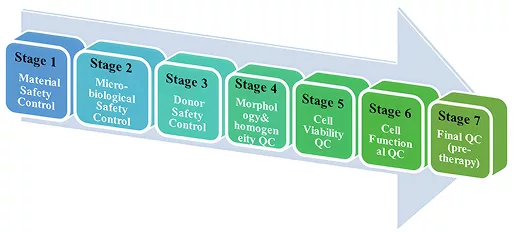 Learn more
Learn more EmCell Stem Cell Center is licensed by the Stem Cell Oversight Committee in Antigua and Barbuda and operates under the Stem Cell Research and Therapy Regulations, 2020, which require the highest standards of compliance with international regulatory guidelines. All of our medical staff are fully registered and licensed.
The administration of stem cell products is conducted under strict regulatory oversight to ensure patient safety. Trained medical professionals follow precise protocols for dosage, delivery method, and monitoring throughout the treatment. Patients’ vital signs — including heart rate, blood pressure, and oxygen levels — are carefully observed during the procedure. All treatments comply with local and international standards, minimizing risks and ensuring that the therapy is delivered accurately, effectively, and safely.
Advanced Cell Therapy Product
Mesenchymal stem cells
Allogeneic stem cells ethically sourced from umbilical cord tissue, a rich source of primitive adult mesenchymal stem cells that retain high proliferative potential.
GMP-compliant manufacturing
GMP (Good Manufacturing Practice) guidelines provide a quality assurance framework for manufacturing cell therapy products intended for clinical use. Adherence to GMP standards ensures the identity, purity, potency, and overall quality of the cell product.
Cultivation protocol
We use preconditioning strategies to enhance the therapeutic function of MSCs. Low passage numbers are maintained to preserve optimal potency and reduce the associated risk of cancer. Our stem cell products are cultured in xeno-free media.
Safety control
Sterility testing is a crucial step in releasing MSC-based cell therapy products. We conduct safety checks at every stage of production, including microbial testing, endotoxin detection, and viral screening using PCR.
Quality control
Each batch undergoes a quality check that includes morphology control, phenotype analysis, proliferative potential, cell viability, differentiation control, and extracellular vesicle (exosome) secretion, all of which indicate the stability of our cell lines.
Final control before clinical use
Before therapy, we perform final viability and quantity checks on our preparations to ensure their quality. These assessments confirm the health, integrity, and functionality of the cells. Our viability.
No risk of rejection
HUCT mesenchymal stem cells are naturally compatible with the body, so there is no risk of rejection and no need for HLA matching.
Ready-to-use
No invasive procedures are required, no stimulation or depletion of the patient’s own body, and no risk of unsuccessful preparation (cultivation). In contrast, our highly effective mesenchymal stem cells are always available in convenient, ready-to-use dosages designed for safe and effective results.
Secretome
Our bodies are regulated by tiny messengers called secretomes — such as exosomes, cytokines, growth factors, and other active molecules. HUCT mesenchymal stem cells are one of the richest sources of these secretomes, naturally supporting growth, repair, and regeneration.
Mechanism of Mesenchymal Stem Cell Action
The therapeutic potential of mesenchymal stem cells (MSCs) for treating various diseases is significant. The
main mechanisms of their function have been widely studied and include the following:

MSCs release growth factors and signaling molecules that reduce inflammation, support healing, and protect cells.
MSCs attract the body’s own stem cells to sites of injury, enhancing tissue repair.
They can differentiate into bone, cartilage, or fat cells to replace damaged tissues.
MSCs can deliver healthy mitochondria to damaged cells, restoring energy and function in neurodegenerative and cardiovascular therapies.
MSCs help balance the immune system, preventing excessive inflammation and tissue damage.
They promote new blood vessel formation to improve oxygen and nutrient delivery.
They provide a scaffold and secrete factors that aid the survival and function of surrounding cells.
MSCs naturally migrate to areas of inflammation or injury, targeting therapy where it is needed.
Stem Cell Products Delivery Methods
Doctors at EmCell Stem Cell Center use various delivery methods to maximize the effectiveness of stem cells in treating different health conditions. The choice of delivery system depends on the condition, the type of stem cell products, and each patient’s individual needs. In this way, we ensure both the efficacy and safety of stem cell therapy.
Stem cell intravenous infusion delivers stem cells directly into the bloodstream, allowing fast absorption and controlled dosing. The cells then circulate and naturally migrate to the areas where repair or healing is needed.
Delivers stem cells into the spinal canal, allowing direct access to the cerebrospinal fluid and central nervous system.
Places stem cells under the skin for slow absorption and gradual release into the body.
Injects stem cells into muscle tissue, supporting local repair and regeneration.
Delivers stem cells directly into joints to reduce inflammation and repair cartilage.
Administers stem cells near the spine to target nerve roots and surrounding tissues.
Introduces stem cells through the respiratory system, reaching the lungs and entering circulation.
Applies stem cells topically to support wound healing, tissue repair, and skin rejuvenation.
Provides a non-invasive route, allowing stem cells to bypass the blood–brain barrier and reach the brain.
Frequently Asked Questions
View some of the most common questions about our treatments, stem cells and clinic.
What are the contraindications for stem cell treatment? Can anyone become a patient?
Are there any side effects?
Multiple peer-reviewed studies have shown that mesenchymal stem cells are a safe cell product. At EmCell Stem Cell Center, we have never had any patients report harmful effects from their infusion or a worsening of their condition.
Common short-term side effects immediately following the cell infusion may include fatigue, headache, and nausea. Patients are under direct medical supervision for the entire treatment and a recovery period of 1–2 hours. We have not observed any side effects.
Can stem cells administered via IV cause clots or embolism?
There is no risk of clot or embolism from intravenous stem cell infusion when proper clinical protocols are followed. Mesenchymal stem cells (MSCs) are generally within an optimal size range (≈17–19 µm), allowing them to pass safely through the pulmonary circulation without blocking blood flow. During production and prior to administration, the cells are processed, washed, and filtered to prevent aggregation, while viability and sterility tests ensure safety. Infusions are administered slowly under strict medical supervision to minimize hemodynamic stress. Serious complications such as embolism are virtually impossible when treatments adhere to established clinical standards.
Can MSCs cause graft-versus-host disease?
No. Unlike other types of stem cells, mesenchymal stem cells (MSCs) do not trigger graft-versus-host disease (GVHD). In fact, MSCs are known for their ability to calm the immune system and reduce the risk of immune-related complications. Many studies report benefits of MSCs in both acute and chronic GVHD. This makes them a safe option even when cells come from a donor.
Do stem cells settle in the lungs?
After IV infusion, mesenchymal stem cells (MSCs) may temporarily pass through the lungs — this is called the “pulmonary first-pass effect.” However, due to their optimal size and strong homing ability, MSCs are not permanently trapped. Instead, they continue on to sites of inflammation or injury in the body while also releasing beneficial molecules in the lungs that support immune balance and healing.
Patient Outcomes
≈85%
Advanced Mesenchymal Stem Cell Treatment has great results in managing a wide range of conditions, relieving pain, and promoting health. ≈85% of our patients report noticeable improvements within the first 3 months after treatment.
- Reduced Inflammation: Decreased systemic and local inflammation, supporting overall health.
- Tissue Regeneration: Repair and regeneration of damaged tissues, such as cartilage, nerves, or muscles.
- Improved Immune Function: Balanced immune responses and reduced autoimmune activity.
- Enhanced Energy and Vitality: Increased stamina, strength, and overall quality of life.
- Better Neurological Function: Improved memory, concentration, coordination, and recovery in neurological conditions.
- Improved Joint and Mobility Function: Reduced pain and enhanced range of motion in joints and muscles.
- Cardiovascular and Metabolic Benefits: Support for heart health, circulation, and metabolic regulation.
NOTE: The effectiveness of MSC-based treatments can vary depending on the patient’s initial condition, underlying disease, age, and lifestyle factors. Improvements often appear gradually, typically within weeks to months after treatment, and may continue over time with proper care. Potential outcomes can range from symptom relief and disease stabilization to significant improvements in overall quality of life.
Learn more about the outcomes you can achieve with our advanced mesenchymal stem cell treatment for your condition.
Worldwide Trust. Real Patients.
Real Results.
Discover how our patients regained health, energy, and quality of life with advanced stem cell therapy. 
Shedule
The duration of treatment may vary depending on the condition, the patient’s medical status, and individual needs. For example, the autism protocol lasts two treatment days and three nights, while most adult protocols include three treatment days and four hotel nights, which are already covered in the price.
- Day 1: Arrival to the beautiful island of Antigua
- Day 2: Medical examination and first treatment session
- Day 3: Second treatment session
- Day 4: Third treatment session and discharge
- Day 5: Departure with renewed strength
Start Your Journey to Health and a Full Life
Here's how to do it
Apply today
You may apply for treatment in any way that is convenient for you. Following a review of your medical form, an online consultation will be scheduled if necessary.
Receive Treatment
We will take care of you from the moment you arrive, guiding you through your life-changing experience with a personalized care plan.
Follow up
After your treatment, our team will follow up with you to monitor your progress, answer any questions, and provide support throughout your health journey.


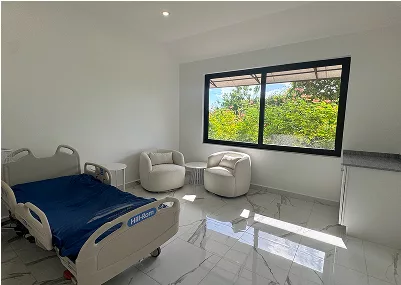

Beautiful, Secure, and Strictly Regulated Location
EmCell Stem Cell Center is located on the beautiful island paradise of Antigua and Barbuda, in the heart of the Caribbean.
We will offer full support, from initial consultation to post-treatment follow-up, including accommodation, island transportation, and VIP airport services. With regular flights from the U.S., Canada, Europe, and the U.K., getting to the island is easy and convenient. Surrounded by natural beauty and tranquility, this is where your journey to a healthier future begins.

Ready to get started?
Complete our brief screening application to take the first step toward the healthier life you deserve with our advanced mesenchymal stem cell therapy.




